92- 01/09/2025 Substandard and counterfeit medicines represent a major and growing threat to global health, undermining patient safety and eroding trust in healthcare systems. This illicit trade is highly profitable—worth an estimated US $65 billion to US $200 billion annually[1]—and affects both emerging and industrialized countries. While the scale of the problem varies by nation, no one is spared.
The World Health Organization (WHO) reports that 1 in 10 medical products in low- and middle-income countries is substandard or falsified[2]. But high-income nations are also affected: in the United States, the Centers for Disease Control and Prevention (CDC) estimated that about 70% of overdose deaths in 2023 involved illegally manufactured fentanyl[3], demonstrating that falsified medicines are not confined to poorer regions.
As the number of cases rises worldwide, patients are emerging not only as victims but also as key agents of change—proactive contributors to safeguarding medicine quality and integrity.
Global health threat
Africa
Africa bears a disproportionate burden. A meta-analysis of 27 studies found that 22.6% of analyzed medicine samples were substandard or falsified, and 34.6% were unregistered[4]. This translates into devastating human costs—up to 500,000 deaths annually in sub-Saharan Africa are linked to poor-quality medicines, including 267,000 from falsified antimalarials and 169,000 from ineffective antibiotics for childhood pneumonia[5].
An investigation by The Guardian and UN News revealed that up to 20% of medicines in Africa are either fake or substandard. These products often contain incorrect dosages, toxic ingredients, or no active ingredients at all—causing preventable deaths, treatment failures, and the erosion of public health.
United States
In the United States, the crisis is also escalating. Counterfeit opioids, stimulants, and even life-saving HIV medicines are increasingly available, driven by illicit online sales and drug trafficking[6].
The French Context
In France, the threat is less widespread but rising—particularly for medicines purchased online. Thousands of unregulated websites are accessible to French consumers, many exploiting a growing culture of self-medication and a desire to bypass prescriptions. Counterfeiters now target not only lifestyle drugs but also treatments for serious diseases. According to the Institute for Research Against Counterfeit Medicines (IRACM), 96% of online pharmacies are illegal. These sites appear to be completely legitimate, and to deceive consumers, some of them use old addresses from the websites of legal pharmacies[7].
Policy Gaps
Adding to the danger is the slow pace of reform. At the 78th World Health Assembly, the WHO delayed updates to the Member State Mechanism (MSM) to combat falsified medical products—pushing coordinated action to late 2025 and beyond.
The roots of the problem lie in a limited number of pharmaceutical suppliers, difficulty in securing timely, verified deliveries, weak independent quality control, multiple, opaque intermediaries in the supply chain. These vulnerabilities create openings for falsified medicines to infiltrate markets worldwide.
Consequences for Public Health and Trust
Substandard or falsified medicines—containing no or wrong active ingredients, incorrect dosages, harmful substances, or deliberately misleading labeling—can:
- Cause treatment failure and disease progression
- Promote antimicrobial resistance
- Increase morbidity and mortality
- Drive up healthcare costs by prolonging illness and requiring repeat treatments
The impact goes beyond individual harm. When patients experience adverse outcomes or deaths linked to poor-quality medicines, entire communities can lose trust in healthcare providers, medicines, and authorities. This crisis of confidence often results in delayed care-seeking, greater reliance on unregulated markets, and weakened public health programs.
Patients as Powerful Agents of Change
Historically, the fight against falsified medicines has been led by regulators, pharmaceutical companies, and global health organizations. But patient organizations—such as the Fight the Fakes Alliance—show that including patient voices adds authenticity, urgency, and reach to awareness and advocacy campaigns. Patients’ action starts with
1. Awareness and Peer Education
Patients can educate themselves and their communities to recognize the risks and warning signs of counterfeit drugs. Campaigns, community talks, and social media initiatives amplify these messages.
Example: In the U.S., ADAP Advocacy and the Partnership for Safe Medicines have produced infographics, blogs, and briefings to help patients spot counterfeit medications.
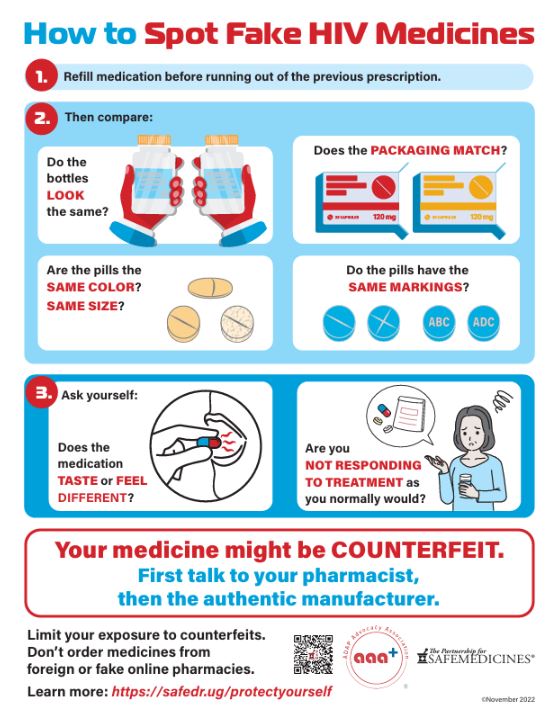
https://www.safemedicines.org/2022/11/patients-are-the-last-line-of-defense.html
2. Reporting Suspicious Medicines
Patients can be frontline detectors by reporting suspect packaging, unusual side effects, or lack of therapeutic effect to pharmacists, healthcare providers, or regulatory hotlines.
3. Advocacy
Patient voices are powerful in influencing government action—pushing for stronger supply chain security, tougher penalties, and greater regulatory transparency. Patient-led campaigns often have more credibility in communities than official statements.
4. Collaboration
Joining international networks such as Fight the Fakes allows patient groups to share experiences, coordinate advocacy, and influence global mechanisms like the WHO’s MSM. They can also support the creation of new bodies, such as the African Medicines Agency, which aims to harmonize regulation and enforcement across Africa.
5. Digital Literacy and Safe Access
Patients can learn to verify legitimate medicine sources, check batch numbers, and use authentication apps where available—reducing reliance on unsafe online sellers.
Patients are not powerless in the face of substandard and counterfeit medicines. Informed, engaged, and organized, they are indispensable allies in the global effort to protect medicine quality and restore trust in healthcare systems. Through their vigilance, education, reporting, communication, and collaboration, patients contribute to protecting others, both locally and globally — and their contribution deserves to be recognized as essential.
Marie-Georges Fayn
[1] World Health Organizations – Atlas of african health statistics. Universal health coverage and the sustainable development goals in the WHO african region, 2018 –https://apps.who.int/iris/bitstream/handle/10665/311460/9789290234135-eng.pdf
[2] Workd Health Organization 1 in 10 medical products in developing countries is substandard or falsified – 28 November 2017 https://www.who.int/en/news-room/detail/28-11-2017-1-in-10-medical-products-in-developing-countries-is-substandard-or-falsified?utm_source=chatgpt.comhttps://www.who.int/en/news-room/detail/28-11-2017-1-in-10-medical-products-in-developing-countries-is-substandard-or-falsified?utm_source=chatgpt.com
[3] US Centers for disease control and prevention – Detection of Illegally Manufactured Fentanyls and Carfentanil in Drug Overdose Deaths — United States, 2021–2024 – 5 December 2024 https://www.cdc.gov/mmwr/volumes/73/wr/mm7348a2.htm
[4] Prevalence of substandard, falsified, unlicensed and unregistered medicine and its associated factors in Africa: a systematic review – 15 Jul 2024 –PubMed
[5] The Guardian – Sun 4 Aug 2024 – Fifth of medicines in Africa may be sub-par or fake, research finds –https://www.theguardian.com/world/article/2024/aug/04/fifth-of-medicines-africa-substandard-fake-research?utm_source=chatgpt.com
[6] Thepartenership for safemedicines, January 2022 Fake HIV medication reached U.S. pharmacies—and patients – https://www.safemedicines.org/2022/01/counterfeit-hiv-medications-in-the-u-s.html
[7]– Réseau Français des Centres Régionaux de Pharmacovigilance (RFCRPV) Achat de médicaments sur Internet : quelles garanties, quels risques ? – RFCRPV https://share.google/3OKL8mVfCWWLHgcry





En postant un commentaire sur www.selfpower-community, vous acceptez les règles de l’espace réaction et reconnaissez à www.selfpower-community la capacité de ne pas publier certaines contributions sans avoir à motiver cette décision.
prendre connaissance des règles